What is 3-Bromo-2-chloro-6-picoline?
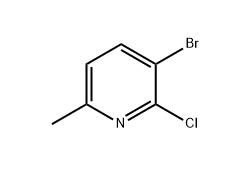
3-Bromo-2-chloro-6-picoline is a halogenated pyridine derivative widely used as a key intermediate in pharmaceutical and agrochemical synthesis. Structurally, it contains a methyl group at the 6-position and both bromine and chlorine substituents on the pyridine ring, giving it high reactivity for further functionalization.
Its combination of electrophilic sites and ring stability makes it an ideal building block for complex fine chemicals, especially those requiring selective halogen exchange or cross-coupling reactions.
How this Intermediate is Produced
Production of 3-Bromo-2-chloro-6-picoline typically involves controlled halogenation of 2,6-dimethylpyridine or related precursors. The process may include:
- Selective bromination at the 3-position using NBS or molecular bromine under catalytic conditions.
- Chlorination using reagents such as thionyl chloride or sulfuryl chloride for high conversion.
- Temperature-regulated reaction steps to maintain positional selectivity and avoid over-halogenation.
- Refinement and purification through distillation or column techniques to meet industrial purity standards.
These methods support large-scale production and ensure consistency required for pharmaceutical intermediates, agrochemical raw materials, and specialty chemical manufacturing.
Features of 3-Bromo-2-chloro-6-picoline
3-Bromo-2-chloro-6-picoline exhibits several features that make it valuable in industrial synthesis:
High Reactivity
Dual halogen groups enhance its reactivity in nucleophilic substitution, Suzuki coupling, or Grignard-type transformations, enabling downstream molecular modifications.
Excellent Selectivity
The substitution pattern provides predictable reaction pathways, reducing by-products and improving atom economy in fine chemical synthesis.
Stable Chemical Profile
Despite its reactivity, the compound maintains good thermal and storage stability, important for long-distance transportation and bulk storage.
Compatibility with Multiple Reaction Systems
It performs well in organic solvents such as toluene, dichloromethane, and DMF, supporting various catalytic systems used in API intermediate manufacturing.
Industries that Widely Use 3-Bromo-2-chloro-6-picoline
This intermediate is used across several sectors due to its adaptable chemical structure:
Pharmaceuticals
It serves as a precursor for active pharmaceutical ingredients (APIs), especially those involving pyridine-based molecular frameworks. Companies producing antiviral agents, anti-inflammatory drugs, and CNS-active molecules often rely on this intermediate.
Agrochemicals
Producers of herbicides, insecticides, and fungicides incorporate this compound as a core building block for heterocyclic pesticide formulations. Its halogenated structure improves biological activity and stability.
Fine and Specialty Chemicals
Its versatility makes it suitable for the synthesis of dyes, catalysts, and complex organic molecules used in material science.
Chemical R&D
Research laboratories favor it for developing novel pyridine derivatives, halogenated intermediates, and structure–activity relationship (SAR) studies.
Handling, Storage, and Safety Guidelines
Proper handling is essential due to its halogenated structure and potential irritant properties.
Handling Precautions
1. Use protective gloves, eyewear, and lab coats.
2. Operate in a well-ventilated area or fume hood to prevent inhalation.
3. Avoid direct contact with skin and eyes.
Storage Requirements
1. Store in tightly sealed containers made of chemical-resistant material.
2. Keep away from heat, oxidizing agents, and direct sunlight.
3. Maintain storage temperature in a cool and dry environment.
Safety and Regulatory Notes
1. Follow SDS guidelines for halogenated pyridine compounds.
2. Dispose of waste according to local environmental regulations.
3. Implement spill-control procedures to prevent environmental contamination.



