1,3-Dibromo-5-tert-butylbenzene is an aromatic compound with two bromine atoms substituted at the 1 and 3 positions of a benzene ring, and a bulky tert-butyl group at position 5. This unique arrangement lends the molecule both reactivity and steric protection, making it a desirable building block in complex organic synthesis.
Often used as a halogenated intermediate, this compound is appreciated for its predictable behavior in cross-coupling reactions and other functional group transformations. Chemists rely on it when they need high positional selectivity and enhanced stability during multi-step reaction schemes.
Basic Properties and Specifications
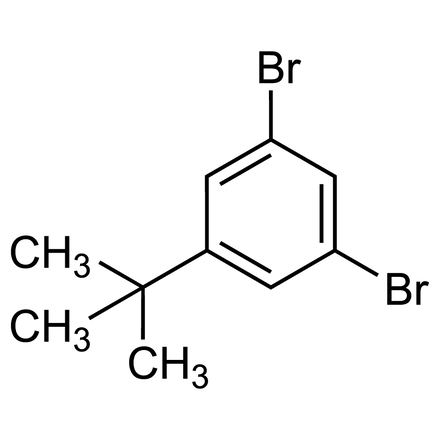
Here are the key identifiers and technical details for 1,3-Dibromo-5-tert-butylbenzene:
▪ Chemical Name: 1,3-Dibromo-5-tert-butylbenzene
▪ CAS Number: 63085-08-3
▪ Molecular Formula: C10H12Br2
▪ Molecular Weight: 308.01 g/mol
▪ Appearance: White to off-white crystalline powder
▪ Purity: Typically ≥98%
▪ Boiling Point: ~290°C (estimated, under reduced pressure)
▪ Melting Point: ~80–84°C
▪ Solubility: Soluble in organic solvents like dichloromethane, chloroform, and toluene
This compound is usually offered in lab-grade and commercial-grade purity, and it can be further customized upon request for high-purity applications.
Applications in Organic Synthesis
1,3-Dibromo-5-tert-butylbenzene finds its niche in both academic and industrial research labs due to its versatile halogenated framework. Common applications include:
Suzuki-Miyaura and Stille Coupling Reactions
The two bromine atoms provide reactive handles for palladium-catalyzed cross-coupling to form biaryl or aryl-alkyl compounds.
Synthesis of Specialty Polymers or Liquid Crystals
The tert-butyl group introduces steric bulk that may influence molecular packing or solubility in polymeric systems.
Preparation of Pharmaceutical Intermediates
Used as a precursor in developing bioactive compounds where controlled substitution on the aromatic ring is critical.
Ligand or Catalyst Development
Serves as a precursor for fine-tuning steric and electronic environments in novel ligand architectures.
Material Chemistry
Useful in the synthesis of functionalized monomers or oligomers in advanced materials and coatings research.
This compound’s symmetrical substitution pattern makes it especially attractive for regioselective reactions where predictability matters.
Handling and Storage Tips
While not classified as highly hazardous, 1,3-Dibromo-5-tert-butylbenzene should still be handled with standard laboratory precautions:
Storage Conditions:
Store in a tightly sealed container in a cool, dry, and well-ventilated area. Avoid exposure to moisture and direct sunlight.
Personal Protection:
Use gloves, lab coat, and safety glasses when handling. Work in a fume hood to avoid inhalation of dust or vapors.
Stability:
Chemically stable under normal conditions. No special stabilizers are required, but avoid contact with strong oxidizing agents.
Proper storage will ensure a longer shelf life and consistent performance in your reactions.
FAQs
Q: Can this compound be used in Suzuki coupling reactions?
Yes, both bromine atoms are reactive under Suzuki conditions. The compound is ideal for forming biaryl linkages.
Q: Is it available in bulk quantities?
Most suppliers offer it in small lab-scale packs (1g, 5g, 25g), but bulk or custom packaging is available upon request for industrial users.
Q: What solvents does it dissolve in?
It dissolves well in organic solvents like dichloromethane, tetrahydrofuran, and toluene. It is poorly soluble in water.
Q: Is the tert-butyl group reactive?
No, the tert-butyl group is generally inert under typical reaction conditions, acting mainly as a steric modulator.
Q: How should it be shipped?
It is typically shipped as a non-hazardous item under standard conditions, but check local regulations for large quantities.
If you're developing halogenated intermediates or designing custom aromatic scaffolds, 1,3-Dibromo-5-tert-butylbenzene deserves a place on your bench.
Need a sample or a bulk quote? Reach out us today for more details or technical support.



