Understanding a specialty chemical like cyclobutane dicarboxylic anhydride can feel intimidating at first, especially if you are new to cyclic anhydrides or fine chemical intermediates. Based on years of hands-on experience working with cyclic anhydrides in both laboratory research and industrial supply chains, I can say that this compound is far more approachable—and far more interesting—than it initially appears.
This article walks you through the essential knowledge, from molecular structure to industrial relevance, in a way that is technically accurate yet easy to follow.
What is Cyclobutane Dicarboxylic Anhydride?
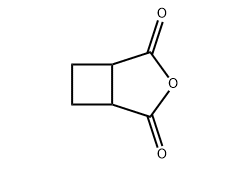
Cyclobutane dicarboxylic anhydride is a cyclic organic anhydride derived from cyclobutane dicarboxylic acid. Structurally, it consists of a four-membered cyclobutane ring bearing two carboxyl groups that have undergone intramolecular dehydration to form an anhydride functional group.
In practical terms, this compound serves as a reactive intermediate rather than a final consumer product. It is valued for its compact ring system, controlled reactivity, and ability to introduce rigidity or functionality into downstream molecules.
From an industry perspective, cyclobutane dicarboxylic anhydride is typically classified as a fine chemical or specialty intermediate, commonly supplied to manufacturers involved in polymers, resins, coatings, or advanced material synthesis.
Molecular Structure and Key Chemical Properties
One of the defining features of cyclobutane dicarboxylic anhydride is its strained four-membered ring. Cyclobutane rings inherently possess higher ring strain than five- or six-membered systems, and this strain subtly influences the compound’s chemical behavior.
Structural highlights include:
1. A cyclic anhydride functional group, which is highly reactive toward nucleophiles
2. A compact molecular geometry, contributing to higher rigidity in derived materials
3. Symmetry that can be advantageous in controlled synthesis and formulation design
In terms of physical properties, cyclobutane dicarboxylic anhydride is generally a solid at room temperature, with moderate thermal stability under dry conditions. Like most anhydrides, it is moisture-sensitive, readily hydrolyzing back to the corresponding dicarboxylic acid in the presence of water.
From experience, proper handling and storage conditions—especially moisture control—play a decisive role in preserving product quality.
Typical Synthetic Routes and Production Considerations
The synthesis of cyclobutane dicarboxylic anhydride typically begins with cyclobutane dicarboxylic acid or a closely related precursor. The key step involves intramolecular dehydration, commonly achieved using chemical dehydrating agents or controlled thermal processes.
In laboratory settings, synthesis routes prioritize purity and reaction control, while industrial production focuses on:
1. Reaction efficiency and yield
2. Minimizing by-product formation
3. Scalability and cost-effectiveness
Industrial manufacturers often optimize reaction parameters such as temperature, catalyst choice, and residence time to balance anhydride formation efficiency with structural integrity.
Based on my experience working with suppliers and end users, consistent quality in cyclobutane dicarboxylic anhydride depends less on exotic chemistry and more on process discipline and purification control.
Reactivity Characteristics and Common Chemical Reactions
The true value of cyclobutane dicarboxylic anhydride lies in its chemical reactivity. Like other cyclic anhydrides, it readily reacts with nucleophiles, making it highly versatile in synthesis.
Common reaction pathways include:
1. Esterification with alcohols, forming mono- or di-esters
2. Amide formation with amines, widely used in polymer and resin chemistry
3. Ring-opening reactions, allowing controlled functionalization
Compared with larger-ring anhydrides, cyclobutane dicarboxylic anhydride often introduces greater rigidity and dimensional stability into the final molecular structure. This characteristic is especially valued in applications requiring mechanical strength or thermal resistance.
For newcomers, understanding these reaction behaviors is the key to using this anhydride effectively rather than treating it as just another reactive reagent.
Applications and Industrial Significance
Cyclobutane dicarboxylic anhydride may not be a household name, but it plays an important role in specialty chemical value chains.
Its primary applications include:
1. Polymer modification, where it enhances rigidity, adhesion, or crosslinking density
2. Resin and coating formulations, contributing to durability and performance consistency
3. Specialty intermediates, particularly where compact cyclic structures are desirable
In comparison with more common anhydrides, cyclobutane dicarboxylic anhydride offers a balance between reactivity and structural contribution, making it attractive for tailored formulations rather than bulk applications.
From a market standpoint, its demand is driven not by volume but by performance-driven use cases, which is why it remains relevant in advanced materials and fine chemical manufacturing.



